NCERT Class 9th Science
CHAPTER 1 MATTER IN OUR SURROUNDINGS
(toc)
In our world, we see many different things that come in various shapes, sizes, and textures. Everything around us is made up of something called "matter," which is what scientists call the stuff that makes up everything in the universe. This includes the air we breathe, the food we eat, rocks, clouds, stars, plants, animals, and even tiny drops of water or grains of sand. All of these things take up space and have weight, which means they have both mass and volume.
Throughout history, people have been trying to understand the world around them. The early philosophers in India divided matter into five fundamental elements: air, earth, fire, space, and water. They believed that everything, whether living or non-living, was made up of these elements. Ancient Greek philosophers had a similar idea about the classification of matter.
Nowadays, modern scientists have come up with two different ways to classify matter based on its physical properties and chemical nature. In this chapter, we will focus on understanding matter based on its physical properties. We will explore the chemical aspects of matter in future chapters.
Physical Nature of Matter
✔MATTER IS MADE UP OF PARTICLES
People used to have different ideas about what matter is like. Some thought that matter is smooth and continuous, like a solid block of wood. Others believed that matter is made up of tiny particles, similar to grains of sand.
To find out which idea is correct, let's do an activity called Activity 1.1:
- Get a beaker that can hold 100 mL of liquid.
- Fill the beaker halfway with water and mark the water level.
- Take some salt or sugar and use a glass rod to dissolve it in the water.
- Pay close attention to any changes in the water level.
- What do you think happened to the salt or sugar?
- Where do you think it went?
- Does the water level change?
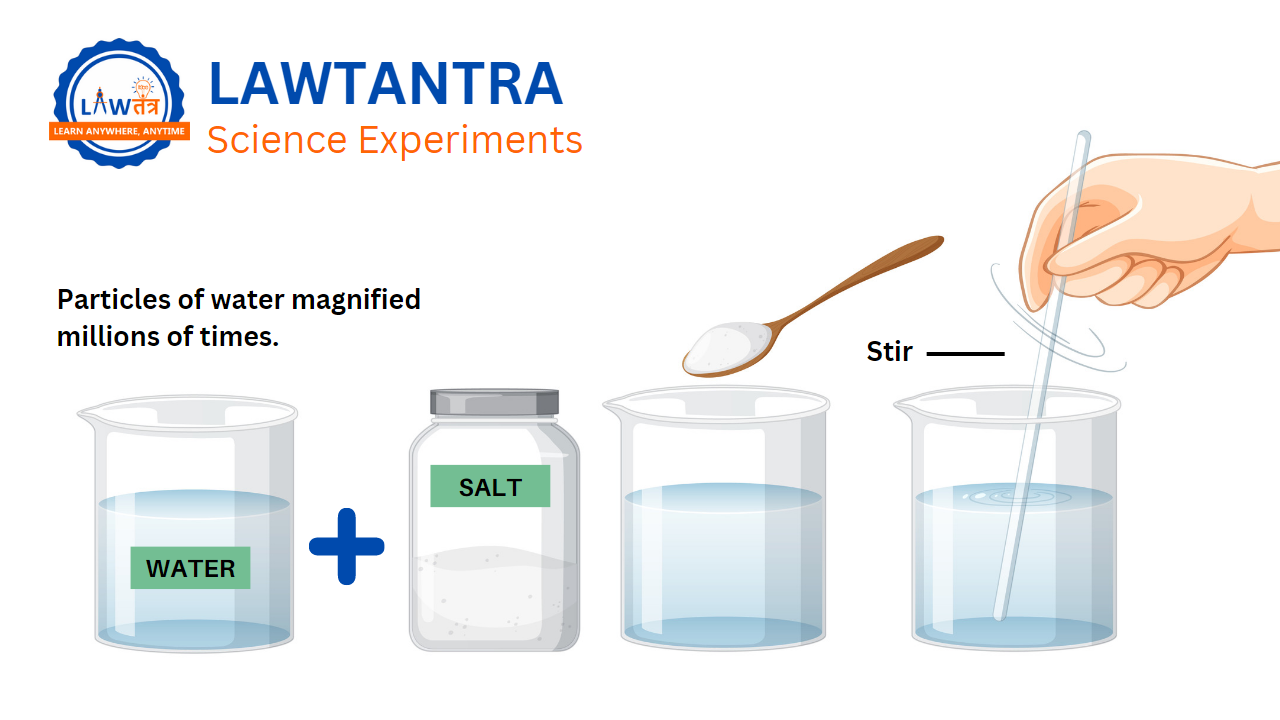
To answer these questions, we need to remember that matter is made up of tiny particles. Whatever was in the spoon, whether it was salt or sugar, has now spread throughout the water. You can observe this in the picture shown in Figure 1.1.
In Figure 1.1, we can see what happens when we dissolve salt in water. The tiny particles of salt go into the empty spaces between the particles of water.
✔HOW SMALL ARE THESE PARTICLES OF MATTER?
Activity 1.2:
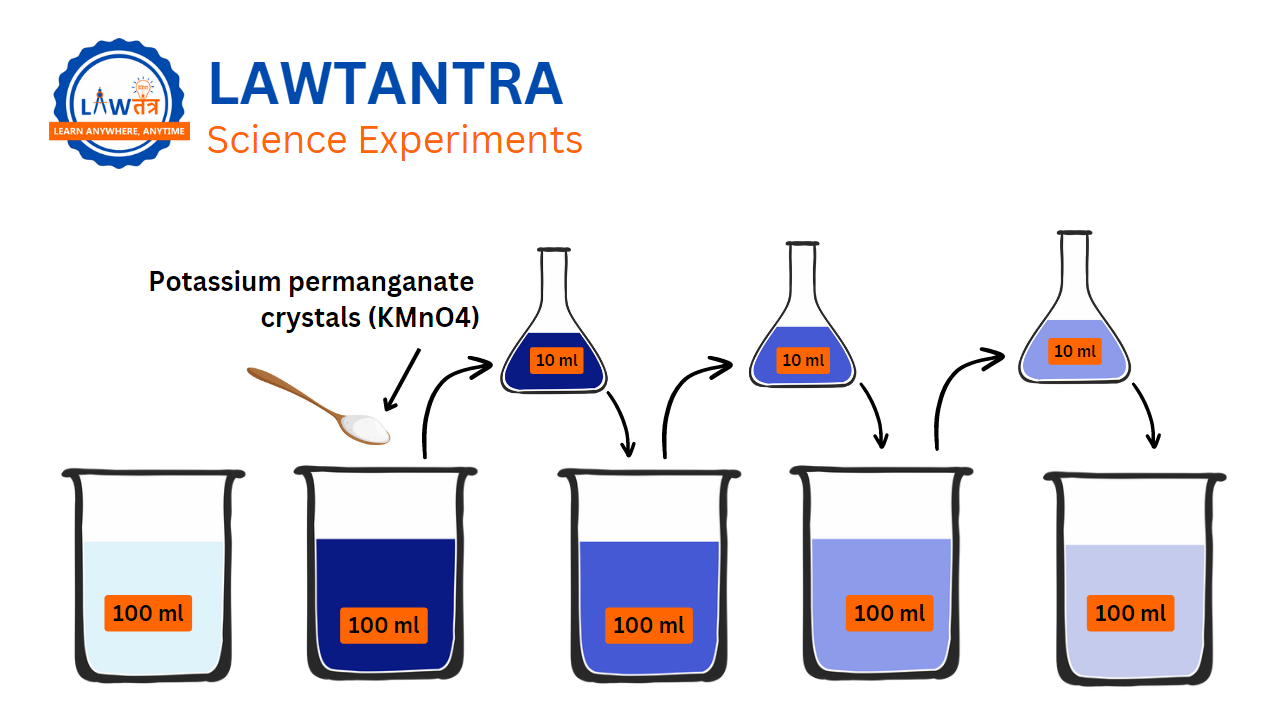
- Take 2-3 small crystals of potassium permanganate and mix them with 100 mL of water until they completely disappear.
- Take out about 10 mL of this solution and mix it with 90 mL of clear water.
- Take out 10 mL of this new solution and mix it with another 90 mL of clear water.
- Keep making the solution weaker by adding more water to it(diluting). Repeat this process around 5 to 8 times.
- Is the water still colored?
In Figure 1.2, we can see how small the particles of matter are. Even
with each dilution, the color becomes lighter, but it is still
visible.
This experiment proves that only a few crystals of potassium
permanganate are enough to color a massive amount of water,
approximately 1000 liters. This tells us that there are millions of tiny
particles in just one crystal of potassium permanganate. These particles
keep dividing into even smaller particles.
You can also try the same activity using 2 mL of Dettol instead of
potassium permanganate. Even after repeated dilutions, you will still be
able to smell it.
Note: The particles of matter are incredibly small - they are so
tiny that it's hard to imagine just how small they are!
Characteristics of Particles of Matter
1.) Particles of Matter: The Space Between Them
In activities 1.1 and 1.2, we noticed that tiny particles such as sugar, salt, Dettol, or potassium permanganate spread out evenly all around the water. The same thing happens when we make tea, coffee, or lemonade(nimbu paani). The particles of one substance mix with the particles of another substance because there is enough space between them.
2.) Particles of Matter: Their Continuous Motion
Activity 1.3:
- Place an unlit incense stick in one corner of your classroom. How close do you need to get to smell it?
- Now light the incense stick. What happens? Can you smell it from a distance?
- Write down your observations.
Activity 1.4:
- Take two glasses or beakers filled with water.
- Be extremely cautious and add a tiny drop of blue or red ink very slowly along the edges of the first beaker. After that, repeat the same process with a drop of honey in the second beaker.
- After adding the drops, let the beakers sit quietly without moving them in your house or in a corner of the classroom. • Record your observations.
- What do you see right after adding the ink drop?
- What do you see right after adding a drop of honey?
- How many hours or days does it take for the ink color to spread evenly throughout the water?
Activity 1.5:
- Take a tiny crystal of copper sulphate or potassium permanganate and drop it into a glass of hot water. Then, take another crystal and drop it into a glass of cold water. Don't mix or stir the water. Allow the crystals to sink and settle at the bottom of each glass.
- What do you see just above the solid crystal in the glass?
- What happens as time goes by?
- What does this tell us about the particles of the solid and the liquid?
- Does the speed of mixing change with temperature? Why and how?
Based on these three activities (1.3, 1.4, and 1.5), we can draw the
following conclusions:
Particles of matter are constantly in motion, which means they have
something called kinetic energy. When the temperature increases, particles
move faster, which means their kinetic energy also increases.
In the three activities mentioned earlier, we observed that particles of
matter mix together on their own. They do this by getting into the spaces
between other particles. This natural mixing of particles from two
different types of matter is called diffusion. We also noticed that when
we heat the substances, diffusion happens even faster. But why does this
happen?
The reason is that when we heat a substance, the particles gain more
energy and move around more quickly. This increased movement makes them
collide and mix with other particles more frequently, speeding up the
process of diffusion. So, higher temperatures increase the speed of
diffusion because the particles move faster and have more chances to
intermix.
3.) Particles of Matter: Attraction between Them
Activity 1.6:
- Play a game in the field with four groups.
- The first group should hold each other from the back, forming a human chain
- In the second group, students should hold hands and form a human chain.
- In the third group, students should touch each other with just their fingertips to form a human chain.
- Then, the fourth group of students should run around and try to break the human chains one by one, separating them into smaller groups.
- Which group was the easiest to break? Why?
- If we consider each student as a particle of matter, in which group did the particles hold each other with the strongest force?
Activity 1.7:
- Find an iron nail, a piece of chalk, and a rubber band.
- Use a hammer to try to break them by hitting them.
- Try to break them by cutting them with scissors or a knife.
- Stretch them and see if they break.
- Now, think about which of these three substances has particles that are held together with a stronger force.
Activity 1.8:
- Find a container, like a cup or a bowl.
- Fill the container with water until it's near the top.
- Now, try to cut the surface of the water using your fingers.
- Were you able to cut the surface of the water?
- Why do you think the surface of water stays together?
Based on the above three activities (1.6, 1.7, and 1.8), we can conclude that particles of matter have forces acting between them. These forces keep the particles together, and the strength of this attraction force can vary from one kind of matter to another.
Questions:
1.) Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, lemon water, smell of perfume.
Answer:
Matter: Chair, air, almonds, cold,
lemon water.
Not matter: Love, smell, hate, thought, smell of perfume.
2.) Give reasons for the following observation:
Answer:
The reason why the smell of hot sizzling food reaches you from several meters away is because the hot food releases a lot of tiny, smelly particles into the air. These particles can travel through the air and reach your nose even when you are some distance away.
On the other hand, cold food releases fewer of these smelly particles into the air. That's why you need to be closer to cold food in order to smell it. The smell of hot sizzling food can reach you from a distance, but for the smell of cold food, you have to get close to it to be able to perceive the smell.
3.) A diver is able to cut through water in a swimming pool. Which
property of matter does this observation show?
Answer:
This observation shows the property of
matter called "density." Water in a swimming pool has a certain density,
and when a diver cuts through it, it demonstrates that the diver is
displacing the water and creating a path through it.
4.) What are the characteristics of the particles of matter?
Answer:
Characteristics of particles of
matter:
- They are very tiny and cannot be seen with the naked eye.
- They are always in constant motion.
- They have spaces between them.
- They attract each other, creating forces of attraction.
- They can be rearranged and separated from each other.





"Thank you for your comment! We value your input and encourage meaningful discussions. However, we kindly request that you refrain from including any spam links in the comment box. Our goal is to maintain a safe and relevant environment for all readers. Your cooperation is greatly appreciated. Thank you!"